In-Clinic Balloon Dilation Procedures – Feasibility and Patient Comfort
By Easmed Academy
Introduction
The first line of treatment for chronic rhinosinusitis is medical therapy, which includes antibiotics, nasal steroids, and saline irrigation. Decongestants, antihistamines, and/or antimucolytics may also be used adjunctively.1,2 Functional endoscopic sinus surgery (FESS) is an option for patients who fail medical management or patients with complex etiologies or comorbidities (e.g., cystic fibrosis, severe polyposis).
Since 2007, balloon dilation technology to treat chronic rhinosinusitis has been available to treat patients with CRS. The minimally invasive technique has gained in popularity due to long-term outcomes that are comparable with FESS. Balloon dilation also offers the advantage of in-clinic procedures under local anesthesia with faster recovery times, reduced postoperative debridements, and less pain medication use.3
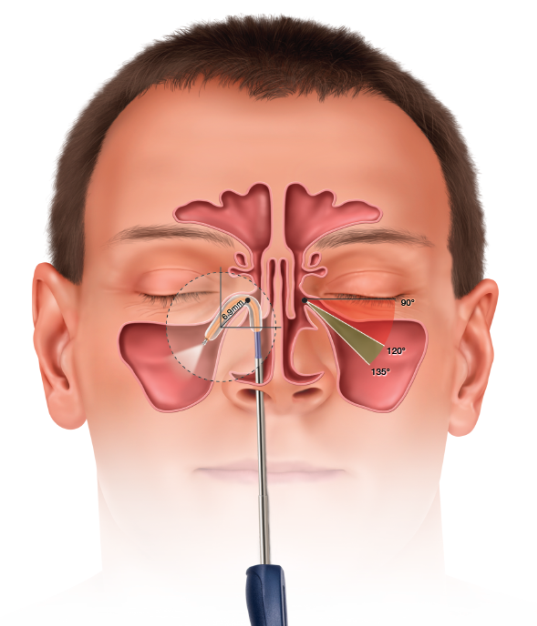
In November 2015, Entellus Medical, now a part of Stryker, received FDA clearance for the XprESS ENT dilation system (XprESS) to access and treat the maxillary ostia/ethmoid infundibula in patients 2 years and older, and the frontal ostia/recesses and sphenoid sinus ostia in patients 12 years and older using a transnasal approach.
Why move from OT to Clinic-based procedures
Rhinology procedures have long been performed successfully in the clinic and this includes diagnostic and surgical interventions such as nasal endoscopy, biopsy, debridement, polypectomy, turbinate reduction, sinusotomy, antrostomy and even ethmoidectomy.4 Balloon dilation of the sinuses and eustachian tube dilation fit right in this group of surgical intervention due to its minimally invasive nature. Movement of these procedures from the operating room into the physicians’ clinic not only provides patients and physicians with a convenient site of service and put less strain on the healthcare system, in terms of freeing up OT time, space and staffs. In addition, it also produces significant cost savings to both patients and the healthcare facility. 5,6
Feasibility of Balloon Dilation Procedures in clinic
Gould et al. have performed a retrospective analysis of prospectively-collected data from adult patients (≥ 18 years of age) who underwent balloon dilation in an clinic-setting between 2007 and 2012.7 Patients with rhinosinusitis unresponsive to aggressive medical management were enrolled across four separate single-arm studies designed to assess procedure outcomes during and after balloon dilation of one or more sinuses. All studies were approved by an Institutional Review Board (IRB) and data were prospectively collected using Good Clinical Practices. Pre-procedure baseline variables and procedure parameters common across all four studies were considered and analyses were performed by an independent statistician. All patients underwent transnasal balloon dilation of the maxillary, frontal, or sphenoid sinus outflow track, or transantral balloon dilation of the maxillary outflow track in the clinic. All study devices were manufactured by Entellus Medical, Plymouth, MN.
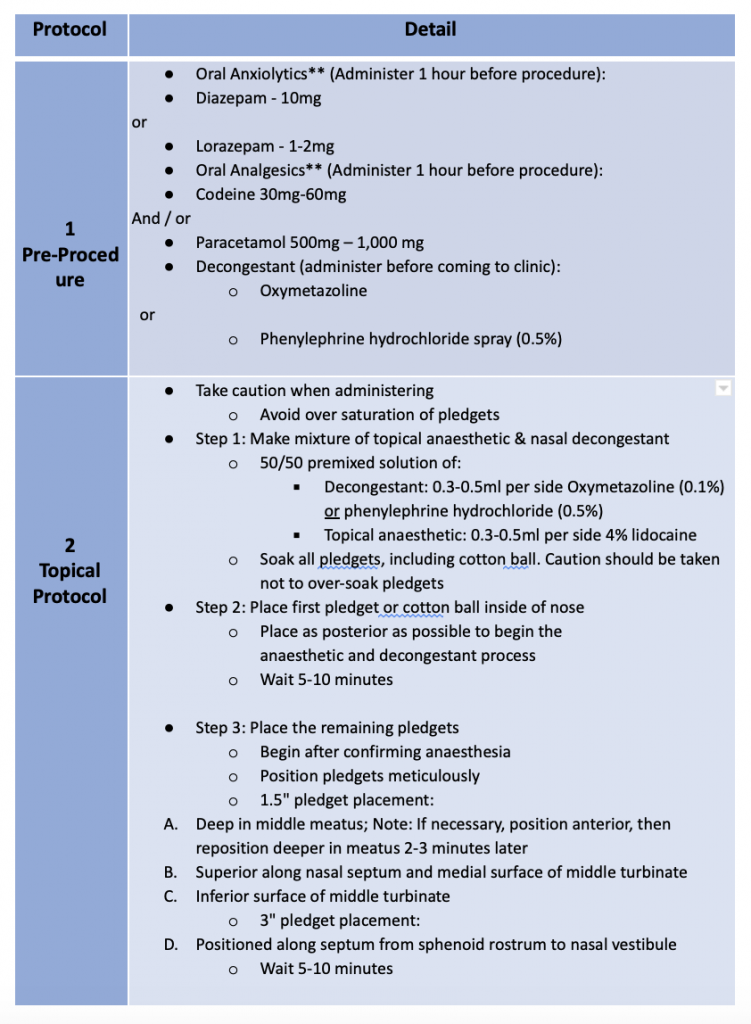
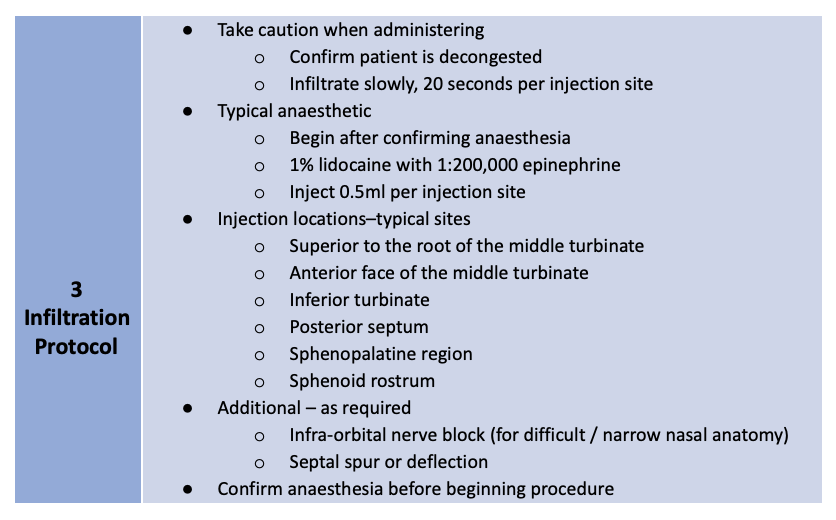
Treatment of the sinuses via transnasal (XprESS™) or transantral (FinESS™) access was determined by the study protocols. Turbinate reduction was allowed in one of the transnasal protocols but no other sinonasal procedures were permitted. A three-step local anesthesia protocol guideline, provided by Dr. Peter Andrews, Consultant Rhinologist, from the Royal National Throat, Nose and Ear Hospital, London, UK are grouped into Pre-procedure, Topical Protocol and Infiltration Protocol as below:
Table 1: Three-step local anesthesia protocol guideline, by Dr. Peter Andrews (disclaimer: this protocol serves only as a guideline and the drugs may or may not be available in your country)
Patient Comfort
Gould et al. further elaborated that Patient comfort and ease of performing the procedure are paramount to successful clinic procedures. 7 Sillers et al. summarized average procedure pain or procedure tolerability from a total of 259 patients across three clinical studies who underwent clinic balloon dilation for chronic rhinosinutis.8 Following procedure completion and prior to discharge from the clinic, each patient was instructed to rate the level of procedure discomfort on a scale from 0 (no pain) to 10 (severe pain).
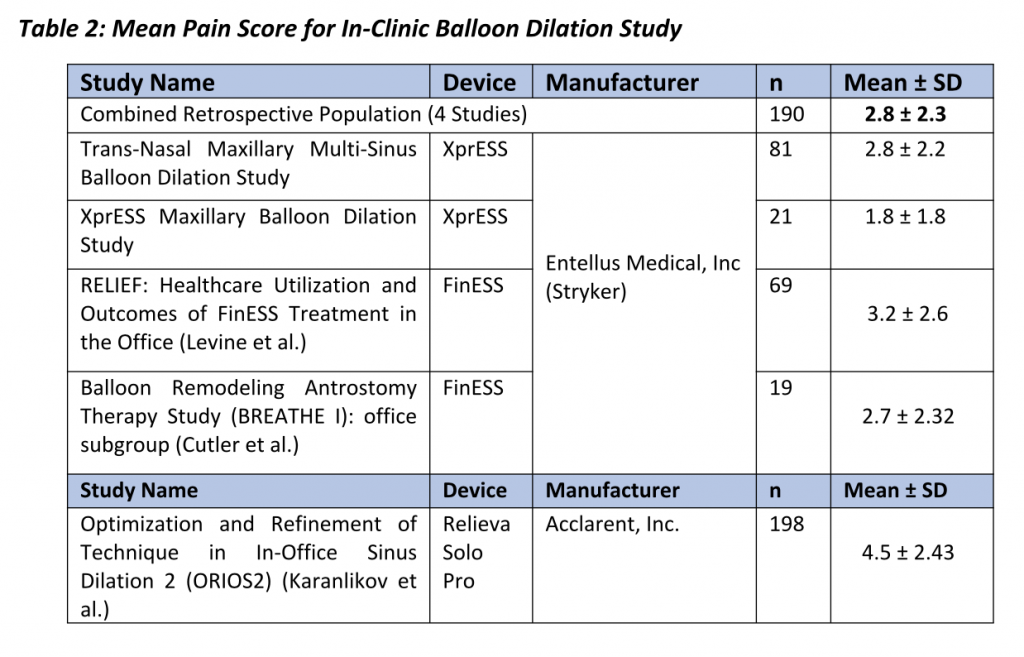
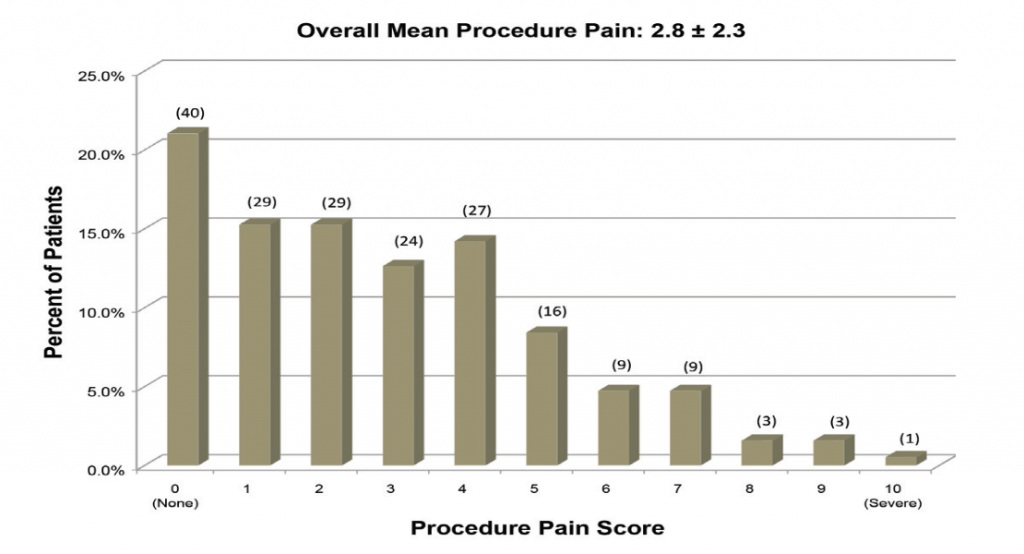
In two studies, patients rated discomfort on a scale of 0 (no pain) to 10 (severe/worst pain) and mean pain scores varied between 2.7 (Cutler et al.) and 4.5 (Karanfilov et al.). Some of these studies also reported the relationship between procedural steps and patient discomfort. Between 40% and 50% of the patients indicated pain was greatest during balloon inflation while 10% to 30% of patients cited insertion of the illumination guidewire or balloon catheter as the most painful part of the procedure. 9,10 Despite evidence to demonstrate balloon dilation is well-tolerated, there is a paucity of data on patient and procedure-related factors that affect patient comfort.
Patients treated by physicians who performed at least 10 study procedures in the office experienced the greatest comfort during balloon dilation. More experienced physicians who performed at least 10 study procedures had an average pain score 1.00 lower than physicians who completed 9 or fewer procedures. Patients without a septal deviation had an average pain score 1.09 lower than those with a septal deviation and patients without allergies had an average pain score 1.05 lower than patients with allergies. Pre-op use of a combination of analgesic and anxiolytic medications is further evidenced by a significant reduction in patient discomfort (p=0.031) in those who received this pre-op regimen versus those who did not.
Conclusion
- In-clinic balloon dilation procedures demonstrates both a high technical success as well as high patient comfort.
- The most important factors affecting patient comfort were the physicians themselves and the procedure volume of each.
- The outcome of the standalone balloon dilation is comparable with the more invasive functional endoscopic sinus surgeries (FESS) at all time points of 6, 12, 18, and 24 months. 3
- In summary, balloon dilation procedures can be safely performed in the physician clinics with good patient’s comfort, given the right patient selection and local anaesthesia guideline.
Interested to start In-Clinic balloon dilation for your patients?
Contact Easmed at customer@easmed.com to arrange for a Hands On Demo session.
Useful links:
References
- Rizzi MD, Kazahaya K. Pediatric chronic rhinosinusitis: when should we operate? Curr Opin Otolaryngol Head Neck Surg. 2014;22:27-33.
- Brietzki SE, Shin JJ, Choi S, et al. Clinical consensus statement: pediatric chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2014;151:542-553.
- Chandra RK, Kern RC, Cutler JL, Welch KC, Russell PT. REMODEL larger cohort with long term outcomes and meta-analysis of standalone balloon dilation studies. Laryngoscope. 2016:124:44-50.
- Armstrong M Jr. Clinic-based procedures in rhinosinusitis. Otolaryngol Clin North Am. 2005;38:1327-38.
- Prickett KK, Wise SK, DelGaudio JM. Cost analysis of clinic-based and operating room procedures in rhinology. Int Forum Allergy Rhinol. 2012;2:207-11.4
- Gould, JD. In-Clinic Balloon Dilation: Procedure Techniques and Outcomes Using a Malleable Multi-Sinus Dilation Tool. ENT Journal. Vendome Healthcare Media. 2012.
- Gould, JD., Brodner D., Atkins J. Patient Comfort During Balloon Dilation Procedures in the Clinic. 2017.
- Sillers MJ, Melroy CT. In-clinic functional endoscopic sinus surgery for chronic rhinosinusitis utilizing balloon catheter dilation technology. Curr Opin Otolaryngol Head Neck Surg. 2013;21:17-22.
- Sikand A. Introduction to an clinic-based sinus surgery technique. Op Techniques in Otolargyngol. 2011;22:246-252.
- Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J Neurosci. 2001;21:9896-903.v
